Small molecule, big potential for treating prostate cancer | MIT News
Prostate most cancers progress is typically pushed by male sex hormones referred to as androgens. Hormone therapy is commonly administered to decrease the degree of androgens in the entire body, but relapse is common when the most cancers cells acquire resistance to these therapies.
A multidisciplinary group of cancer scientists led by Angela Koehler, the Samuel A. Goldblith Career Development Professor in Used Biology and a member of MIT’s Koch Institute for Integrative Most cancers Investigation, has discovered a compact molecule that can selectively goal a important protein involved in the stabilization of androgen receptor molecules.
People with sophisticated varieties of prostate most cancers are most generally handled with androgen deprivation remedy, which lowers the degree of male hormones these kinds of as testosterone via surgery or treatment. On the other hand, patients invariably relapse from these therapies and create castration-resistant prostate most cancers (CRPC).
The following line of protection depends on medications these kinds of as abiraterone or enzalutamide, which block testosterone and relevant androgens from binding to androgen receptors. Androgen receptors are proteins in just cells that bind testosterone and ultimately upregulate the expression of genes that engage in a central job in prostate cancer development. Roughly half of CRPC patients answer to second-line medication, even though most relapse inside of just one to two many years.
Through a wide variety of means, most cancers cells can acquire other mechanisms to activate the androgen receptor pathway, regularly by expressing variant forms of androgen receptors that function even in the absence of androgens. There is no acknowledged little molecule drug that can inhibit androgen receptor variants, leaving couple productive therapeutic selections for CRPC people.
In a review appearing in Cell Chemical Biology, the staff identified a extremely selective inhibitor of CDK9, which was not only extremely strong versus CRPC in cell traces and mouse types, but may have broad applicability to assortment of cancers.
“Resistance to treatment is a widespread obstacle in most cancers care,” suggests postdoc Shelby Doyle PhD ’20, who co-led the examine as a portion of her graduate thesis work in biological engineering. “Our compound could enable beat that resistance and assist us fully grasp no matter whether there is a more resilient approach to chemotherapy in prostate most cancers.”
Binding results
Medication that block androgen receptor exercise function by focusing on the ligand binding area. Analogous to a lock and key, the ligand binding domain presents a well-outlined structure into which the small molecule drug can suit. But androgen receptor variants are missing the ligand binding area, rendering androgen receptor-blocking medications ineffective.
These “undruggable” androgen receptor variants are challenging to review with regular procedures. Common binding assays, which test tiny molecules from a purified protein of fascination, count on the existence of well-defined protein structures like a ligand binding domain to perform. But when purified, androgen receptor variants deficiency these kinds of composition. What’s more, the purification course of action strips goal proteins of involved cofactors, molecules that interact intently with the concentrate on protein and hence could serve as oblique targets.
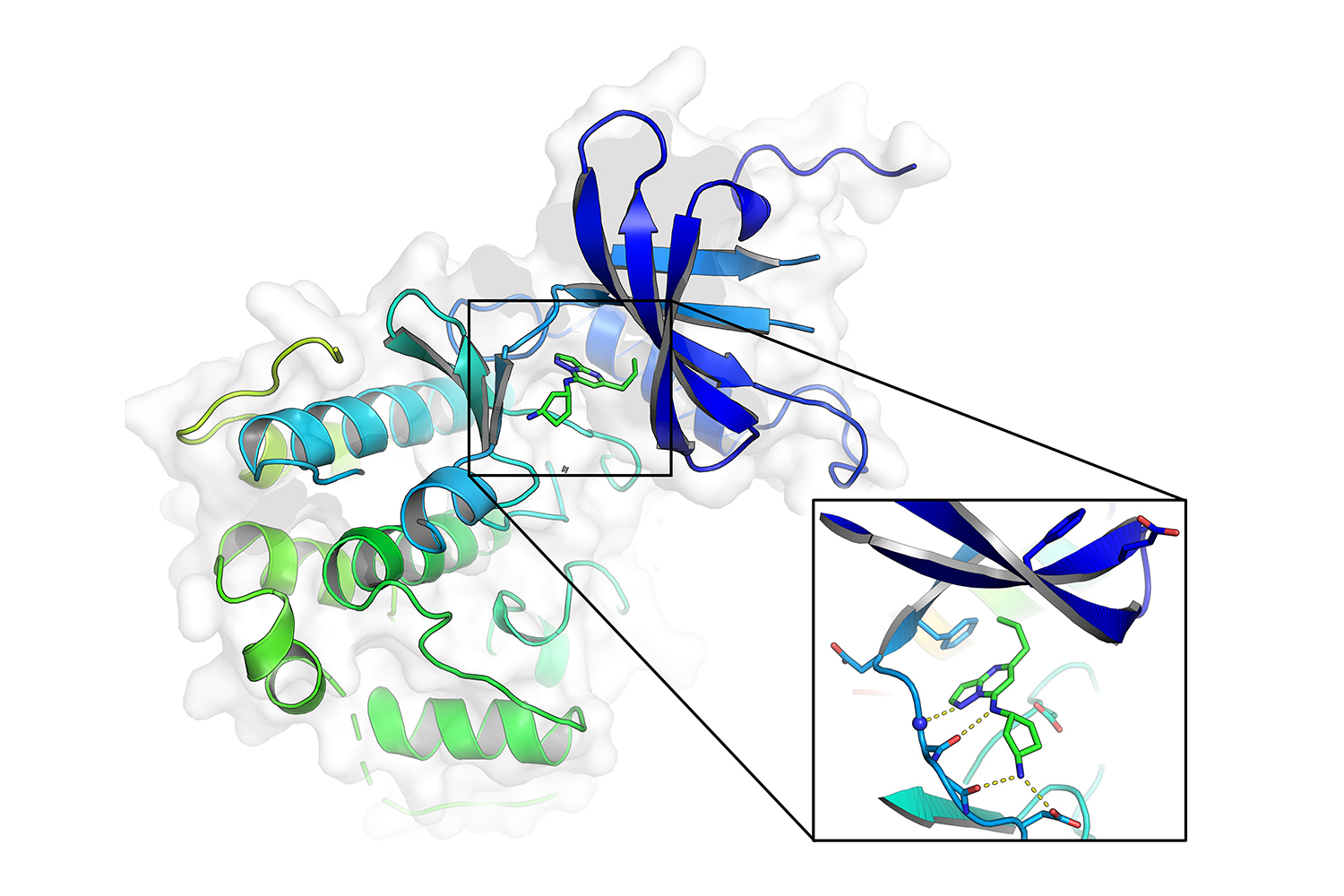
The Koehler lab specializes in modest-molecule microarray (SMM) platforms that display screen libraries of modest molecules from proteins alongside one another with their suites of cofactors. The cofactors themselves not only current further targets, but their presence signifies that the androgen receptor variant molecule is extra probable to undertake a defined structure, related to that which it requires in the mobile. Making use of the SMM platform to monitor a library of 50,000 compounds for interactions with androgen receptor variants, scientists have been equipped to identify a handful of binders. A single of them, KI-ARv-03, proved to be pretty powerful versus CRPC mobile lines.
“Our direct compound, KI-ARv-03, was especially remarkable to us because it was in a position to reduce androgen receptor exercise by decreasing stages of both androgen receptor and the chemoresistance-connected variant of androgen receptor in CPRC styles,” claims Doyle.
Experiments unveiled that KI-ARv-03 does not goal the androgen receptor protein specifically, but binds to a person of its cofactors, cyclin-dependent kinase 9 (CDK9). CDK9 is just one of a household of proteins that regulate a range of significant mobile processes, this sort of as the mobile cycle or gene transcription, and performs a position in stabilizing the androgen receptor molecule. By inhibiting CDK9, KI-ARv-03 destabilizes androgen receptor proteins and curbs the expression of oncogenes.
A recipe for optimization
CDK9 inhibitors have revealed promise as opportunity most cancers therapies, due to the fact they exploit a vulnerability one of a kind to most cancers cells, recognized as oncogene dependancy. Most cancers cells can get over disordered or dysfunctional pathways by appreciably raising the transcription of particular genes that enable them to survive and develop. Nonetheless, oncogene dependancy leaves most cancers cells vulnerable in a way healthful cells are not: If these oncogenic pathways are inhibited even for a short period of time, the most cancers cells die.
Regardless of this guarantee, CDK9 inhibitors have had limited scientific achievements, probably in element due to the fact they bind to many associates of the CDK spouse and children and outcome in significant degrees of toxicity. Even so, the researchers found that KI-ARv-03 is selective for CDK9 to an unusually higher diploma, and is not likely to interact with other users of the CDK household.
“Kinase inhibitors usually focus on many kinases since of the higher structural similarity of these proteins, and it is quite challenging to style selective binders for these,” explains investigate scientist André Richters, a chemist in the Koehler lab who co-led the examine with Doyle. “Multiple rounds of chemical optimization and retesting are ordinarily needed to make improvements to selectivity although retaining efficiency. Achievement, however, is not guaranteed. The discovery of an extremely-selective and concurrently potent kinase inhibitor these as KI-ARv-03 as a direct consequence of screening is very uncommon.”
Kronos Bio, an MIT spinout co-started by Koehler, produced a more effective model of the CDK9 inhibitor, KB-0742. Using proprietary technologies, the firm has optimized the KI-ARv-03 compound to make it a lot more powerful, although retaining the authentic selectivity uncovered by the MIT workforce. Preclinical exams in cell traces and mouse designs expose significantly decreased tumor development in CPRC designs and other oncogene-addicted cancers as opposed to common chemotherapy.
The MIT group is learning the chemistry guiding KI-ARv-03’s selectivity as very well as the system of motion for cell state disruption. They hope their conclusions will have wide applicability across a lot of most cancers forms, specifically people whose malignancy is also sustained by oncogene habit and dysregulated transcriptional systems.
“Prostate most cancers is not one of a kind in its resistance to treatment,” points out Koehler, “nor is it the only tumor form with a described oncogene addiction. Researching the biochemical habits/qualities of KI-ARv-03 and evaluating it to other compounds uncovered by our screens will help us generalize our results to other cancer forms and enhance enhancement of other therapeutics.”
This work was supported, in component, by the Koch Institute-Dana-Farber/Harvard Most cancers Heart Bridge Undertaking, the Koch Institute Support (main) Grant, the National Institutes of Health, the Royal G. and Mae H. Westaway Loved ones Memorial Fund, the Ono Pharma Basis, the MIT Middle for Precision Most cancers Medicine, and Janssen Pharmaceuticals, Inc., by way of the Transcend partnership with the Koch Institute.







