Amazing Video Reveals a New Kind of Leidenfrost Effect We’ve Never Seen Before
Over 193 °C (379 °F) anything almost magical happens to water in a pan.
Known as the Leidenfrost outcome, when you sprinkle drinking water on to a sizzling area, the drops float above the area on a layer of vapor. They adhere all over for a instant or two longer than they would at a reduce (but however above boiling) temperature, skittering throughout the pan in advance of evaporating absent.
This transpires to all diverse forms of liquids, as extensive as the temperatures are much hotter than every individual liquid’s boiling position. But scientists have just learned one thing even more intriguing – that this influence can come about even involving two droplets of different liquids, leading to them to ‘bounce’ off each other.
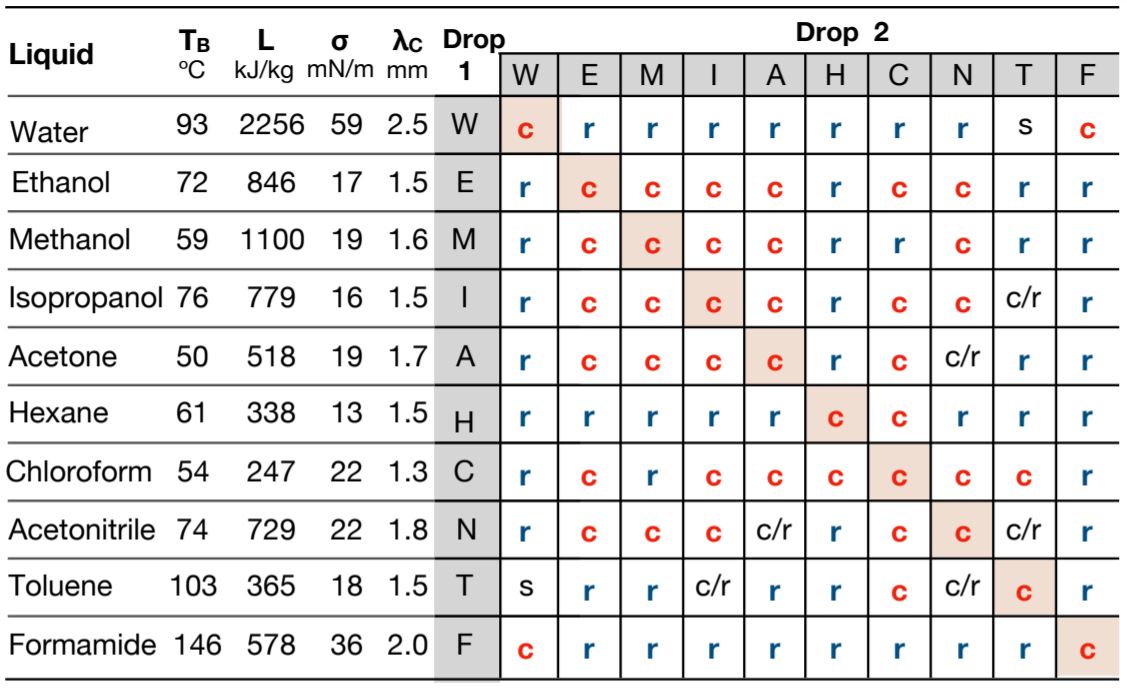
The staff of researchers, led by initial creator, College of Puebla physicist Felipe Pacheco-Vázquez, looked at liquids these types of as h2o, ethanol, methanol, chloroform, and formamide, and analysed whether or not two drops of each and every mixture of liquids would ‘coalesce’ straight away into a single droplet, or would ‘consecutive rebound’ (bounce off every other a couple instances).
They did this by working with a little steel plate with a slight inward slope and heating it to 250 degrees, which was properly above any of the liquids’ boiling factors (which ranged from acetone’s 50 °C to formamide’s 146 °C at the lab’s altitude).
A large obvious fall of one particular liquid was then additional with a little blue dyed drop and they viewed what occurred. Some – when each drops were the identical sort of liquid or liquids with related boiling details – just merged straight away, when they slid into just about every other at the lowest position of the plate.
Some others took their time ahead of merging. They appeared a great deal like the compact droplet bouncing on the big a person. You can see this amongst ethanol (the little droplet) and drinking water (the significant droplet) under in the online video:
https://www.youtube.com/enjoy?v=sqWzHzhAE8o
“The direct coalescence lasts some milliseconds, and it was noticed mainly with drops of the exact same liquid (e.g. drinking water-drinking water) or liquids with identical attributes (e.g. ethanol-isopropanol),” the staff writes in a new paper.
“In distinction, drops with substantial variances in homes (e.g. water-ethanol or drinking water-acetonitrile) continue being bouncing in the course of a number of seconds, or even minutes, although they evaporate right up until achieving a significant measurement to at last coalesce.”
Ultimately right after the liquid that evaporates quicker shrinks to a particular dimension, the two drops incorporate and then ‘pop’ – you’ve got received just one marginally even bigger mixture of liquids skating all over as a substitute of two.
You can see from the desk underneath no matter if any of the two liquids coalesced (c), rebounded (r), did some blend of each (c/r), or in distinctive cases remained as divided phases mainly because they are not able to be mixed (s).
The crew recommend that this bouncing is truly a ‘triple Leidenfrost effect’, the place the drops don’t just conclusion up with an insulating vapour layer from the surface area of the incredibly hot plate, but also concerning the two droplets.
“The bouncing dynamics is manufactured due to the fact the drops are not only in Leidenfrost state with the substrate, they also experience Leidenfrost outcome between them at the moment of collision,” the team writes.
“This transpires owing to their various boiling temperatures, and therefore, the hotter fall functions as a very hot surface area for the drop with decreased boiling stage, producing a few contact zones of Leidenfrost state concurrently. We known as this state of affairs the triple Leidenfrost effect.”
The study has been released in Physical Overview Letters.




