To make mini-organs grow faster, give them a squeeze | MIT News
The closer men and women are bodily to just one yet another, the bigger the prospect for trade, of items like suggestions, data, and even an infection. Now researchers at MIT and Boston Children’s Clinic have discovered that, even in the microscopic natural environment in just a one mobile, bodily crowding will increase the prospect for interactions, in a way that can noticeably change a cell’s wellbeing and development.
In a paper posted right now in the journal Mobile Stem Mobile, the researchers have proven that bodily squeezing cells, and crowding their contents, can bring about cells to develop and divide a lot quicker than they normally would.
While squeezing one thing to make it develop might sound counterintuitive, the group has an clarification: Squeezing functions to wring drinking water out of a mobile. With much less drinking water to swim in, proteins and other mobile constituents are packed closer jointly. And when sure proteins are brought in shut proximity, they can bring about mobile signaling and activate genes in just the mobile.
In their new analyze, the scientists discovered that squeezing intestinal cells activated proteins to cluster along a particular signaling pathway, which can enable cells keep their stem-mobile state, an undifferentiated state in which can rapidly develop and divide into more specialized cells. Ming Guo, affiliate professor of mechanical engineering at MIT, states that if cells can just be squeezed to boost their “stemness,” they can then be directed to rapidly construct up miniature organs, these as artificial intestines or colons, which could then be applied as platforms to have an understanding of organ operate and test drug candidates for different conditions, and even as transplants for regenerative medicine.
Guo’s co-authors are guide writer Yiwei Li, Jiliang Hu, and Qirong Lin from MIT, and Maorong Chen, Ren Sheng, and Xi He of Boston Children’s Clinic.
Packed in
To analyze squeezing’s effect on cells, the researchers blended different mobile types in alternatives that solidified as rubbery slabs of hydrogel. To squeeze the cells, they put weights on the hydrogel’s floor, in the form of both a quarter or a dime.
“We preferred to obtain a important total of mobile sizing transform, and those people two weights can compress the mobile by one thing like ten to thirty % of their full volume,” Guo clarifies.
The group applied a confocal microscope to evaluate in 3D how specific cells’ shapes adjusted as just about every sample was compressed. As they predicted, the cells shrank with tension. But did squeezing also influence the cell’s contents? To remedy this, the researchers initial appeared to see no matter if a cell’s drinking water content material adjusted. If squeezing functions to wring drinking water out of a mobile, the researchers reasoned that the cells need to be much less hydrated, and stiffer as a result.
They measured the stiffness of cells just before and just after weights ended up used, applying optical tweezers, a laser-based approach that Guo’s lab has utilized for years to analyze interactions in just cells, and discovered that in fact, cells stiffened with tension. They also saw that there was much less motion in just cells that ended up squeezed, suggesting that their contents ended up more packed than regular.
Up coming, they appeared at no matter if there ended up alterations in the interactions among sure proteins in the cells, in reaction to cells remaining squeezed. They focused on quite a few proteins that are recognized to bring about Wnt/β-catenin signaling, which is associated in mobile growth and routine maintenance of “stemness.”
“In general, this pathway is recognized to make a mobile more like a stem mobile,” Guo states. “If you transform this pathway’s exercise, how most cancers progresses and how embryos produce have been proven to be quite distinct. So we believed we could use this pathway to exhibit how mobile crowding is vital.”
A “refreshing” path
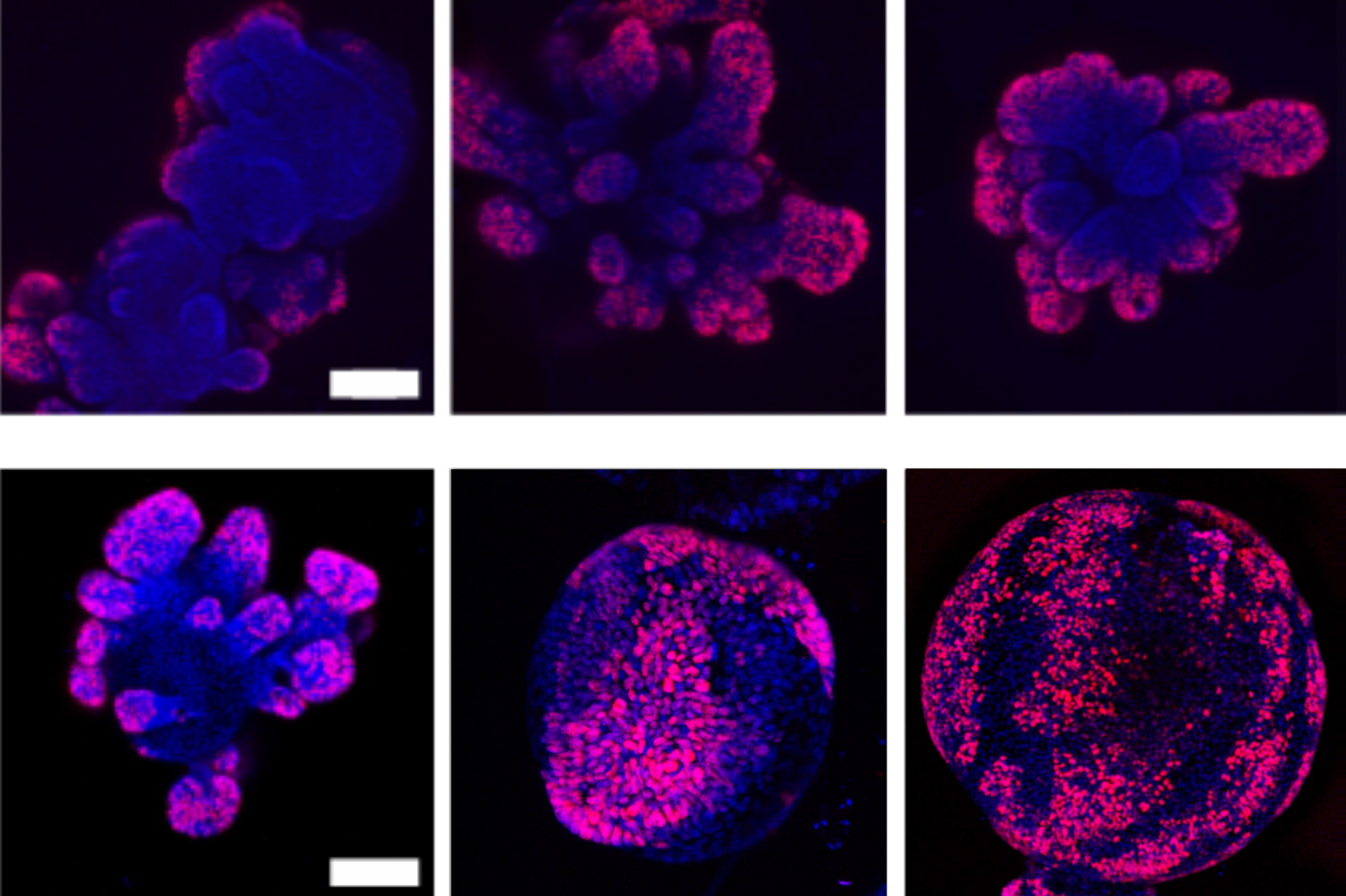
To see no matter if mobile squeezing has an effect on the Wnt pathway, and how speedy a mobile grows, the researchers grew modest organoids — miniature organs, and in this scenario, clusters of cells that ended up gathered from the intestines of mice.
“The Wnt pathway is notably vital in the colon,” Guo states, pointing out that the cells that line the human intestine are regularly remaining replenished. The Wnt pathway, he states, is vital for sustaining intestinal stem cells, generating new cells, and “refreshing” the intestinal lining.
He and his colleagues grew intestinal organoids, just about every measuring about half a millimeter, in quite a few Petri dishes, then “squeezed” the organoids by infusing the dishes with polymers. This inflow of polymers increased the osmotic tension surrounding just about every organoid and pressured drinking water out of their cells. The group noticed that as a result, particular proteins associated in activating the Wnt pathway ended up packed closer jointly, and ended up more most likely to cluster to flip on the pathway and its growth-regulating genes.
The upshot: People organoids that ended up squeezed really grew larger sized and more rapidly, with more stem cells on their floor than those people that ended up not squeezed.
“The big difference was quite clear,” Guo states. “Whenever you use tension, the organoids develop even even larger, with a ton more stem cells.”
He states the final results exhibit how squeezing can influence a organoid’s growth. The findings also demonstrate that a cell’s habits can transform based on the total of drinking water that it has.
“This is quite general and wide, and the opportunity affect is profound, that cells can just tune how a lot drinking water they have to tune their organic effects,” Guo states.
Going forward, he and his colleagues approach to check out mobile squeezing as a way to speed up the growth of artificial organs that scientists might use to test new, personalized drugs.
“I could consider my very own cells and transfect them to make stem cells that can then be formulated into a lung or intestinal organoid that would mimic my very own organs,” Guo states. “I could then use distinct pressures to make organoids of distinct sizing, then attempt distinct drugs. I think about there would be a ton of options.”
This study is supported, in part, by the Nationwide Cancer Institute and the Alfred P. Sloan Foundation.




